The video covers the basic lewis structures youll see in an introductory chemistry class. For each unshared electron pair draw 2 small dots right next to each other around the central atom.
Show the electron geometry of the central atom.
How to draw covalent lewis dot structures.
Draw the simple structure skeleton structure of the compound by connecting everything.
Total n c f v n b2 where v number of valence electrons for the atom.
For each single bond draw a line going away from the atom.
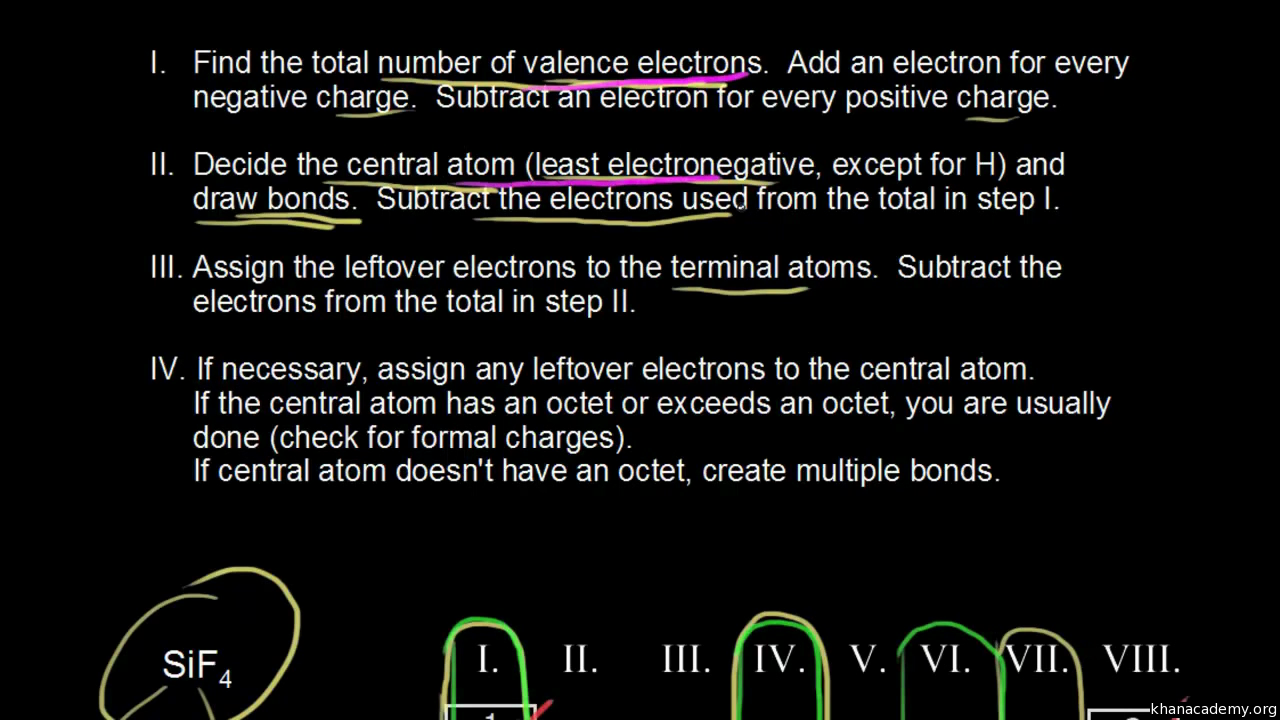
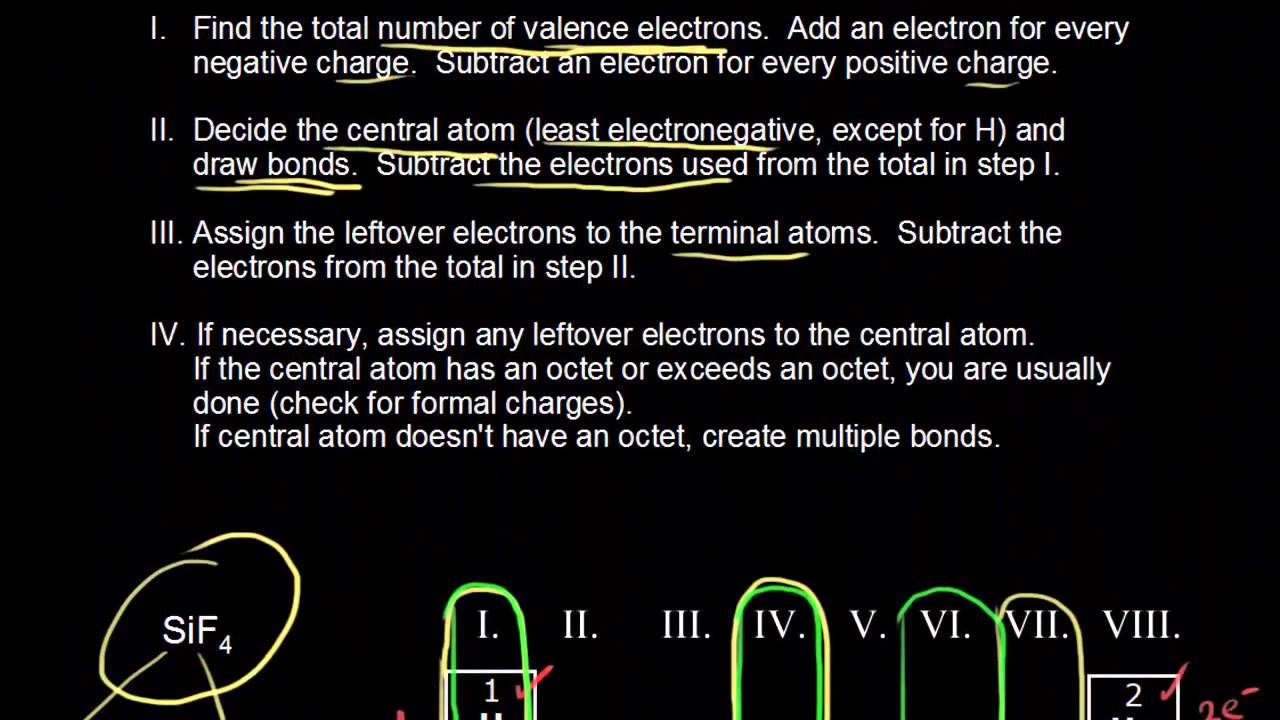
These instructions outline the kelter strategy to draw lewis structures for molecules.
Follow these simple steps to correctly draw a lewis dot structure.
For double and triple bonds instead of 1 line draw 2 or 3 respectively.
A video tutorial for how to draw lewis structures in five steps.
Find the total number of valence electrons in this step add up the total number of valence electrons from all the atoms in the molecule.
Add electrons to all the noncentral atoms.
Put any unused.
Calculate the formal charge of the ligand atoms to complete the lewis structure.
Add up the total number of valence electrons found in the entire compound.
Get more chemistry help at wwwbreslyn.
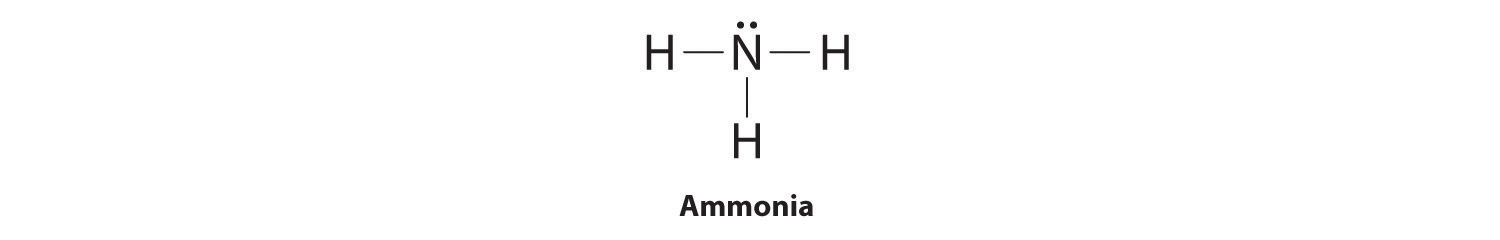
These non bonding valence electrons are called lone pairs of electrons and should always be indicated in lewis diagrams.
Constructing lewis electron structures.
These valence electrons are negatively charged and are attracted to the positively charged nucleus made up of neutrons and protons.
The lewis dot structure is a visual which represents the outermost shell of electrons also known as valence electrons and possible covalent bonds within an atom or molecule.
The following procedure can be used to construct lewis electron structures for more complex molecules and ions.
Add together the valence electrons from each atom.
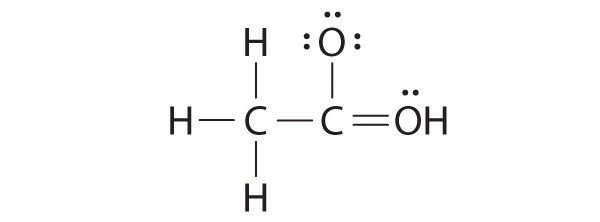
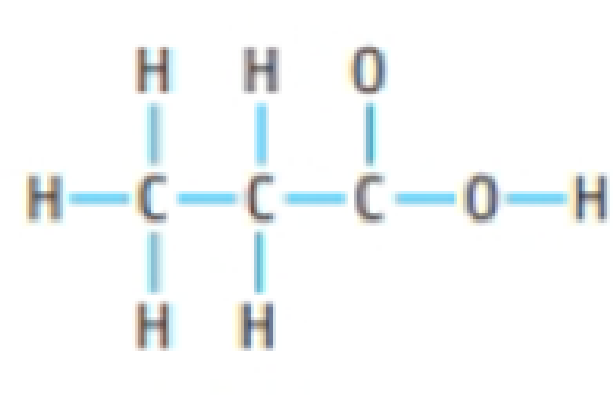
Lewis structure of acetic acid acetic acid ch 3 cooh can be written out with dots indicating the shared electrons or preferably with dashes representing covalent bonds.
Determine the total number of valence electrons in the molecule or ion.
Draw lewis structures for covalent compounds.
If the central atom formal charge is zero or is equal to the charge on the species the provisional electron distribution from 4 is correct.
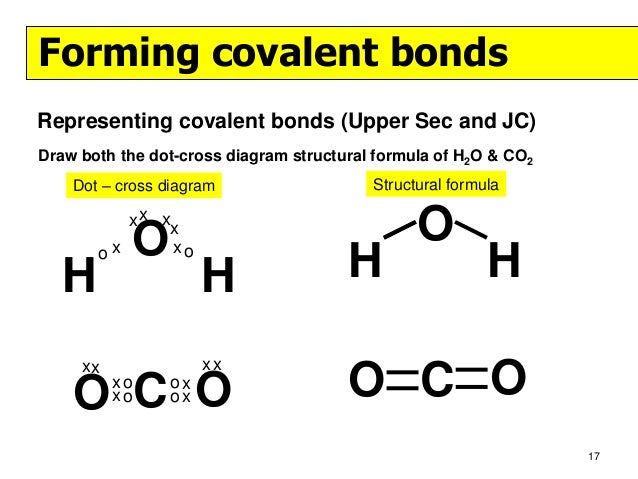
Lewis diagrams aka lewis structures lewis dot structures lewis dot diagrams are useful because they use simple drawings to show how atoms share valence electrons in molecules polyatomic ions.







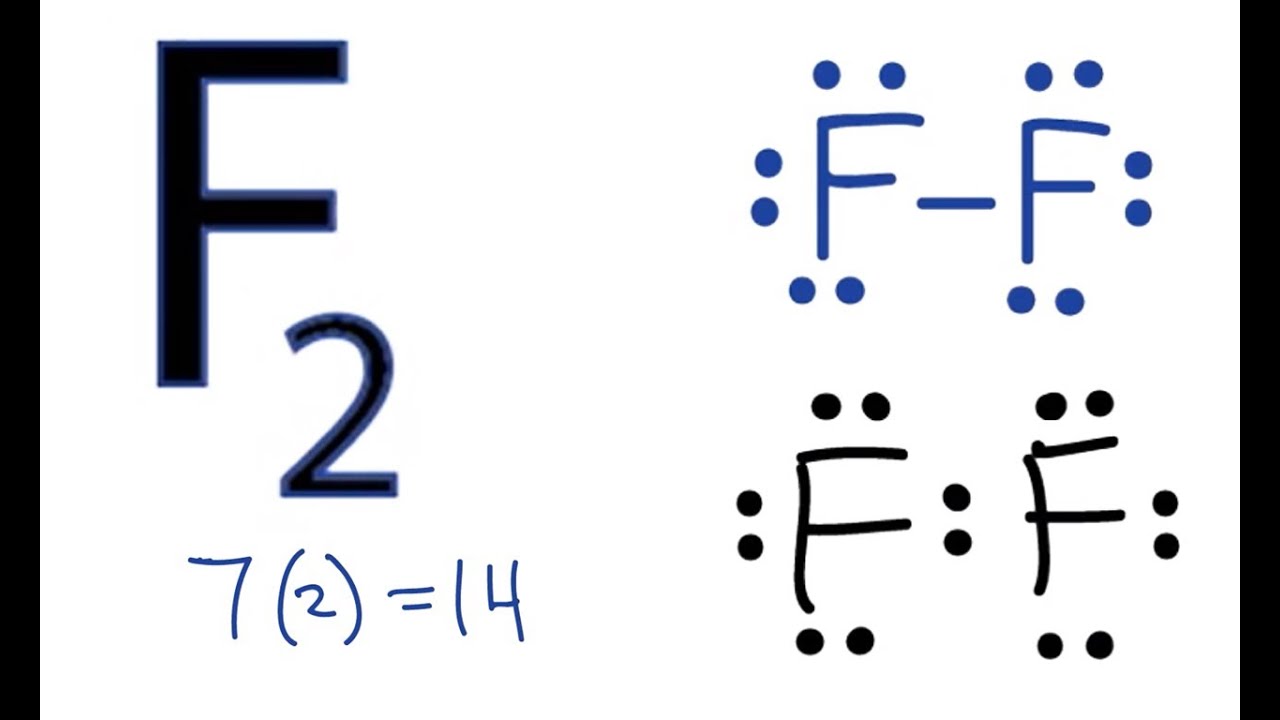


No comments:
Post a Comment